Nitrogen is the most common limiting nutrient for canola production. Therefore, a good understanding of the nitrogen cycle, nitrogen’s role in canola growth and development, and nitrogen management options are needed to maximize canola production, profitability and sustainability, with reduced production risk.
Important tips for nitrogen management
- Ensure an adequate supply of nitrogen is applied to support crop yield potential.
- High-yielding hybrid cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More will require more nitrogen to support yield, but may be more efficient at extracting nitrogen from soil.
- Follow the 4R Nutrient Stewardship principles (for fertilizer applications) of always applying nutrients at the Right rate with Right timing, using the Right source and the Right placement.
- Banding is the most efficient placement method for nitrogen in Prairie conditions, but adequate seed and fertilizer separation must be ensured.
- Seed-placed fertilizer guidelines were developed when researchers and growers were seeding at eight pounds per acre. Since that time, growers have adopted lower seeding rates. New hybrid varieties with larger and more vigorous seed may be somewhat more tolerant to stress, including effects of fertilizer placed in the seed row, but this may not be a worthwhile risk with low initial seed populations and better placement options available.
Role of nitrogen in canola plants
Nitrogen is the most common limiting nutrient (other than water) for canola production. Therefore, a good understanding of this nutrient is needed to efficiently manage nitrogen fertilizer and maximize economic returns.
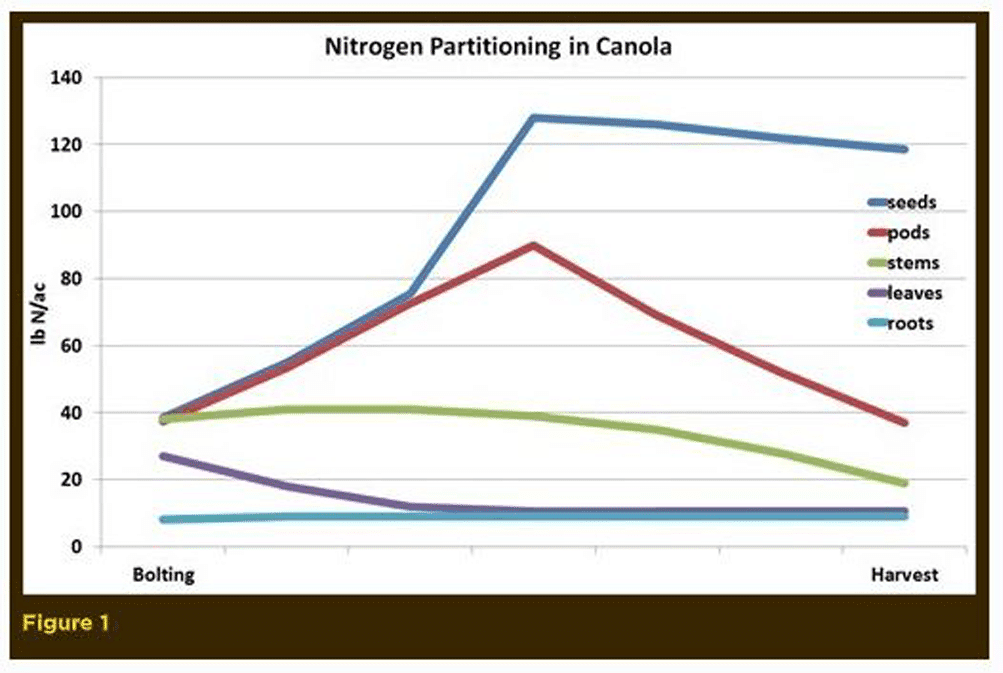
Canola, like most crops, contains large amounts of nitrogen, which is a part of many critical plant components, including amino acids and proteins (which may form enzymes), genetic material (nucleotides and nucleic acids) and components found in membranes (such as amines) and co-enzymes (among others). The majority of the nitrogen in green plant tissue is present as enzyme protein in chloroplasts where chlorophyll is located. By harvest, the majority of the nitrogen in a canola plant is found as seed protein. The relative nitrogen proportions in the plant changes over time and growth stage. The nitrogen proportioning closely resembles the dry matter partitioning as shown in Figure 1.
The nitrogen level in canola plants is highest in the early seedling stage when young leaves are the majority of the plant’s dry matter. As the plant grows and reaches the flowering stage, the overall nitrogen level declines due to leaf loss. By maturity, canola straw contains just 0.5 to 1.5 per cent nitrogen while the seed contains 3.4 to 4 per cent nitrogen.
Nitrogen effects on canola growth
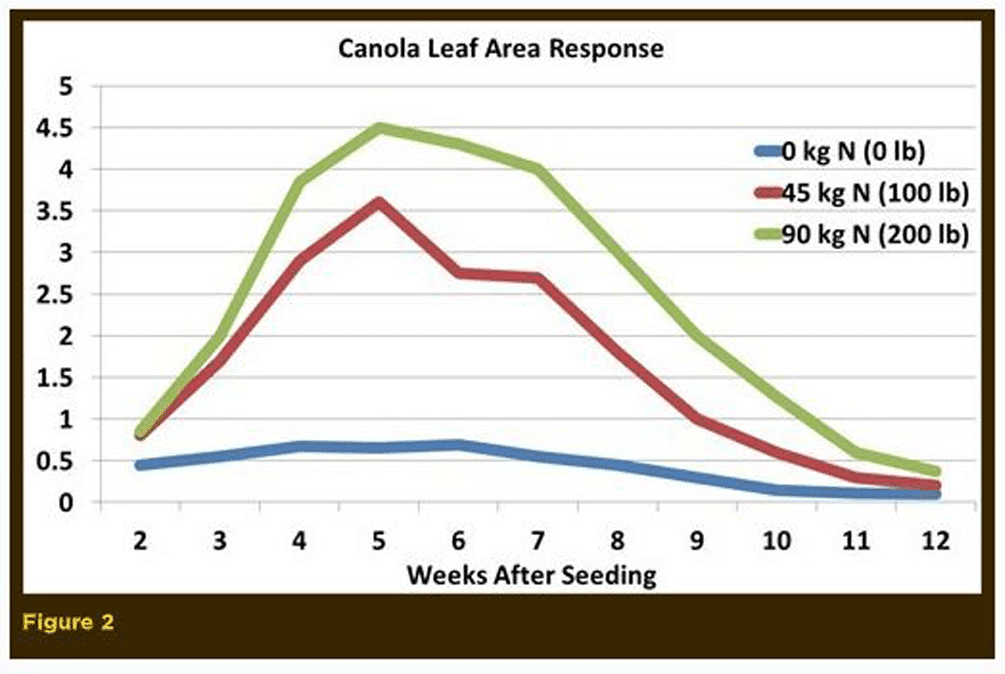
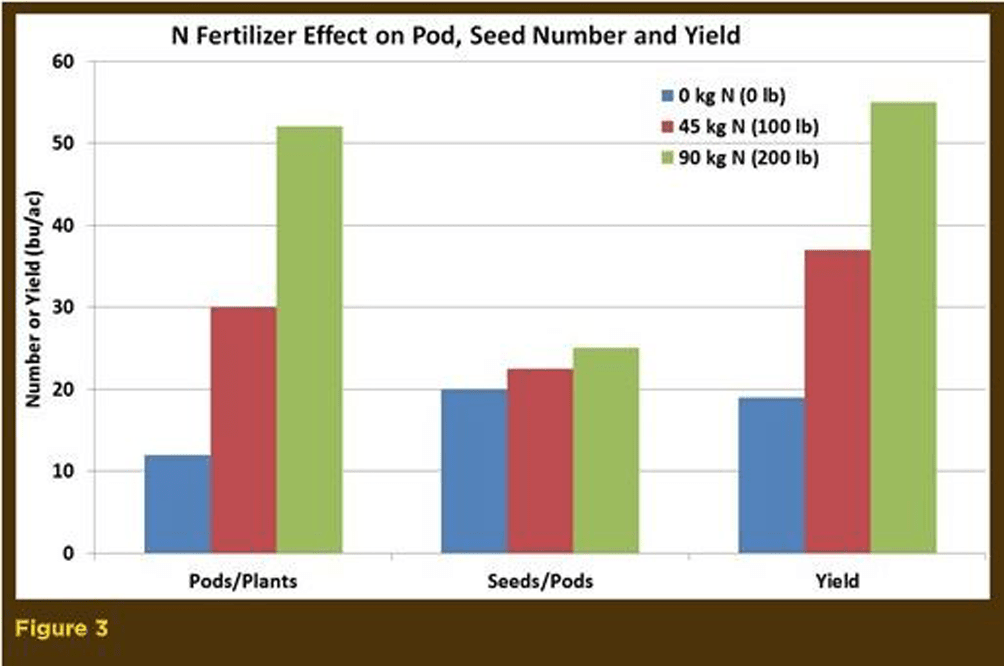
The most obvious impact of nitrogen is an overall increase in plant growth (height and dry matter). This stimulation occurs early in the vegetative stage and continues into the reproductive stage. Research from England illustrates the canola leaf stimulation, leaf area and pods/seed production by nitrogen fertilizer (Figures 2, 3, 4).
Several western Canadian studies have examined seasonal plant growth and nutrient uptake of canola. In general, nutrient uptake increased with time at early growth stages and reached a maximum four weeks after emergence. Total nitrogen accumulation in canola plants was highest nine to 10 weeks after emergence.
Nitrogen fertilizer mainly increases canola leaf area index, leaf duration, plant weight, growth rates, number of flowering branches, plant height, number of flowers, number and weight of pods and seed yield. Therefore, good nitrogen fertility early in the season is necessary to produce a large, photosynthetically efficient leaf area that will support high numbers of flowers, pods and seed yield.
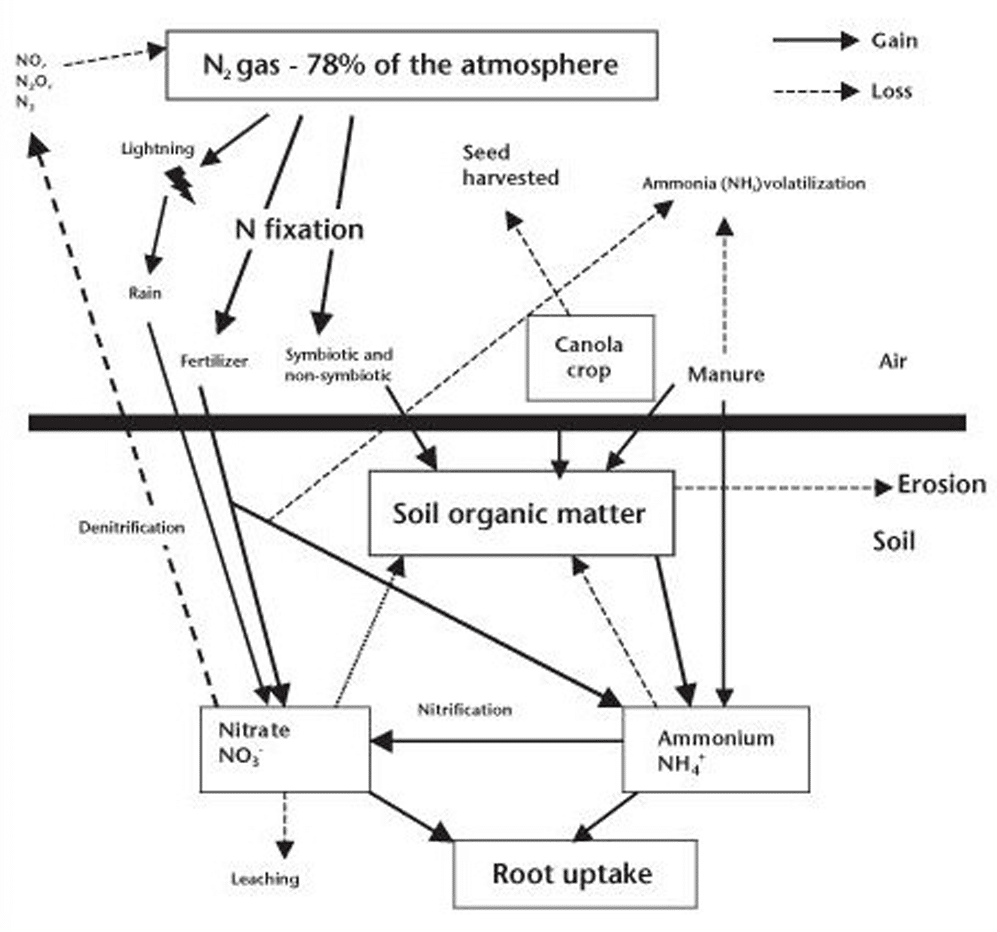
Nitrogen cycling
Increasing plant-available nitrogen
Mineralization
Soil organic matter is a major nitrogen reservoir, containing several thousand pounds of organic nitrogen per acre. This large organic nitrogen storehouse needs to be decomposed by soil microbes before becoming available for root uptake. The decomposition process is called mineralization.
In the first step, ammonium is changed to nitrite (NO2-). In the second step, nitrite is change to nitrate. Under normal soil conditions, the bacterial oxidation of ammonium to nitrite is much slower than nitrite to nitrate. Therefore, very little nitrite is normally found in soil.
This is fortunate because nitrite is toxic to plants. The result of these rate differences is that most of the plant available nitrogen in the soil tends to be nitrate. This is also why most soil testing labs analyze soil for nitrate and don’t include ammonium.
Since soil microbial activity drive these nitrogen transformations, soil conditions such as temperature, moisture and acidity will strongly affect the rates at which these processes occur. The net amount of mineralization also depends on the level of organic matter. Greater soil organic matter content means the greater amount of nitrogen that can be mineralized. If soil is cold, saturated or very acidic, then mineralization and other microbial activities will proceed slowly. Cultivation stimulates organic matter decomposition and mineralization of nitrogen by improving aeration, the breakdown of soil aggregates, and physical mixing that gives soil microbes access to new organic matter supplies.
Long-term no-tillA type of tillage management technique where soil is undisturbed from harvest through planting, seeding with narrow knives/hoes or discs. More is beneficial for nitrogen cycling, and the increase in potential mineralization under favourable climatic conditions. A comparison of canola yields grown on adjacent fields found the area that had been in no-tillA type of tillage management technique where soil is undisturbed from harvest through planting, seeding with narrow knives/hoes or discs. More for 31 years had 16 per cent higher yield than the nine-year no-tillA type of tillage management technique where soil is undisturbed from harvest through planting, seeding with narrow knives/hoes or discs. More field. These higher grain yields may be achieved without necessarily having to add additional crop inputs (fertilizer).
Nitrogen fixation
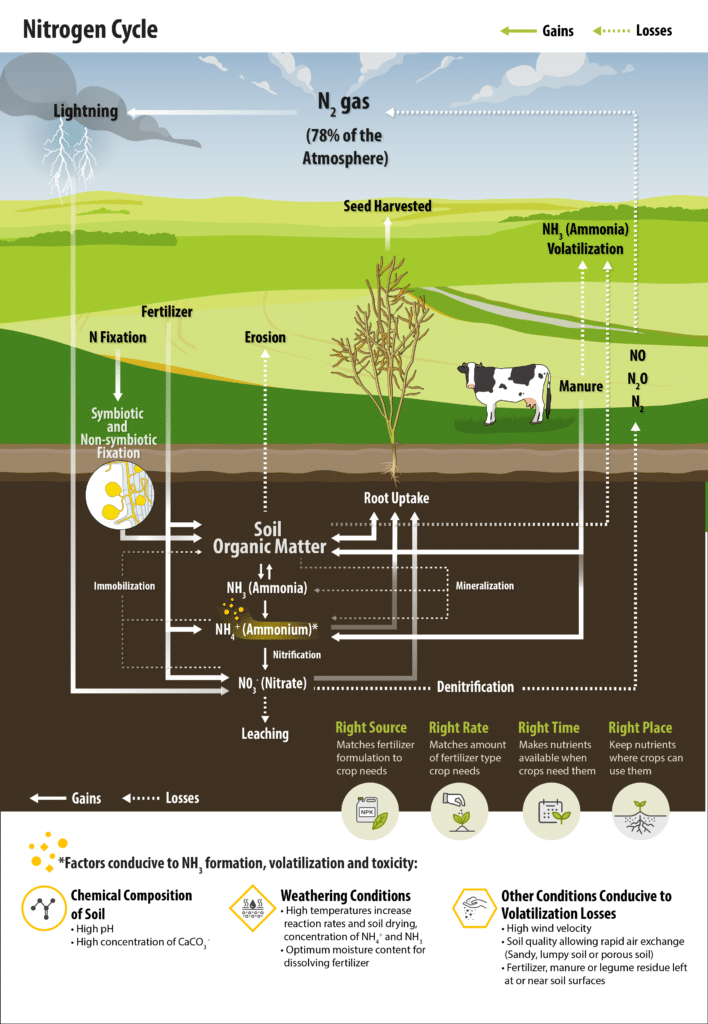
There are several sources of plant available nitrogen for the soil system, in addition to soil organic matter decomposition and fertilizer additions. The atmosphere contains 78 per cent nitrogen (N2) and thus is a huge source of nitrogen. But it must be changed to plant available forms first, through biological or industrial processes called nitrogen fixation. Biological fixation of atmospheric nitrogen (or dinitrogen gas, N2) is performed by certain bacteria or blue-green algae species. Biological nitrogen fixation in soil falls into three general types:
- symbiosis with legumes
- associative
- free-living nitrogen fixing bacteria
Symbiotic nitrogen fixation is much larger relative to the other types of fixation. Canola is not a legume and cannot form the symbiosis with rhizobia to fix atmospheric nitrogen. However, canola can benefit from residual nitrogen fixed by previous legume crops.
Associative nitrogen fixation occurs when bacteria just inside the root, or on the root surface, use root exudates for energy to fix atmospheric nitrogen. The plant benefits indirectly when the bacteria dies and its nitrogen is released through mineralization. The results of many global experiments on cereal inoculation with associative nitrogen fixing bacteria have shown varying and unpredictable responses. This suggests that the interactions between plants and the bacteria are complex, unstable and can vary greatly depending on genotypes. Limited research on canola inoculation using associative nitrogen fixing bacteria shows that some strains will successfully colonize the roots of Brassica species, but often do not significantly influence harvest dry weight or nitrogen accumulation.
Reducing plant-available nitrogen
Several different mechanisms contribute to nitrogen loss from soil, and therefore, lost opportunity for increasing yield. Understanding the conditions that promote such losses can be valuable in avoiding such conditions in the field and improving the nitrogen use (and fertility) efficiency.
Denitrification
One major nitrogen loss from soil occurs through a microbial process called denitrification. Denitrification occurs regardless whether the nitrate source is from fertilizer, manure or decomposition. Under good management practices, usually is less than 10 per cent on Prairie soils.
Certain bacteria can change nitrate to gases such as nitrous oxide (N2O) and atmospheric nitrogen (N2), which escape back to the atmosphere. These soil bacteria have the ability to switch their respiration from using oxygen to nitrate or sulphate. Since respiration is more efficient using oxygen, these denitrifying bacteria will only switch to nitrate or sulphate if oxygen is absent, such as in waterlogged soil. Therefore, denitrification becomes significant when soils become saturated. Other secondary factors that encourage denitrification include carbon availability (crop residues), warm soil, and neutral to alkaline pH.
On the Prairies, combinations of warm and saturated soil conditions are not that common. In spring, although there may be saturated conditions, it is usually cold so microbial activity is low. In the summer, soils are warm but not usually saturated.
Research on the Prairies has shown that nitrogen losses due to denitrification tend to be during spring thaw and they can be considerable. A study near Edmonton, Alberta found that 16 to 60 per cent of the total amount of nitrogen lost annually due to denitrification was lost immediately following snowmelt. (Note: this does not mean that 60 per cent of total soil nitrogen was lost, just that up to 60 per cent of the losses that occurred in that time period). During spring thaw on the Prairies, frozen subsoil is often a barrier for water drainage and the overlying thawed soil becomes saturated.
The second major period of denitrification loss occurs in late spring and early summer during rainfall events that cause soil saturation. Denitrification occurs regardless of the nitrate source (fertilizer, manure or decomposition). For this reason, effective nitrogen fertilizer management strives to avoid having large amounts of nitrate present during spring melts. Summerfallow is prone to large denitrification losses, since large amounts of nitrate and moisture are stored during the fallow year, which increases the denitrification potential in the next spring thaw.
Immobilization
Immobilization is the second major nitrogen transformation that reduces the plant available nitrogen supply. Soil bacteria may use either nitrate or ammonium for their own growth, temporarily tying up the nitrogen in the soil organic storehouse. Immobilization is essentially the reverse of mineralization, and occurs when residues with low nitrogen content (relative to the carbon content, resulting in a high carbon to nitrogen ratio), such as straw from cereal crops, are being decomposed. Since these residues don’t contain enough nitrogen for the microbes to make their own protein, they need to use the nitrate and ammonium from the soil solution as a nitrogen source. Therefore soil microbes compete with plant roots for the available nitrogen, and plant growth suffers when nitrogen supplies are inadequate for both microbial and plant growth needs. The poor crop growth that can be observed in heavy chaff rows is partly due to the immobilization of nitrogen and other nutrients by the decomposing microbes. To reduce these immobilization losses, the fertilization strategy of placing the fertilizer away from residues (e.g. banding) can be utilized.
Note that immobilization is only a temporary loss, since nitrogen that is tied up this way will later become available (after the microbes due and decompose).
Leaching
Nitrate is a form of nitrogen present in the soil solution which is not adsorbed to the soil and moves readily with soil water. As a result, nitrate leaching can sometimes occur. Leaching losses can be significant in sandy soils with high rainfall or under summerfallow, but overall leaching likely contributes to less than 10 per cent of the available nitrogen losses on the Prairies. To reduce leaching losses, time fertilizer applications to avoid prolonged exposure to wet conditions, and consider band placement of ammonium or ammonium-producing sources to delay the conversion to the vulnerable nitrate form.
Summerfallow is especially prone to denitrification losses since large amounts of nitrate and moisture are stored during the fallow year, which increases the denitrification potential in the next spring thaw. Summerfallow is also a contributor to nitrate leaching losses. However, research showed that an application of 20 inches of water was necessary to move nitrogen below the 24 inch depth in sandy soil that was summerfallowed 1.
Volatilization
Volatilization occurs when ammonia escapes from the soil to the atmosphere. Such losses can happen in a number of ways. One obvious loss occurs when anhydrous ammonia fertilizer is improperly applied into a soil that is too dry, or into soil too wet for furrows to seal properly behind the openers. Broadcasting urea fertilizer on the surface without incorporation can also lead to significant volatilization losses if significant rainfall (more than 12 millimetres or 0.5 inch) does not occur soon after application. All ammonium-based fertilizers are subject to volatilization if broadcast on the surface of soils with high pH, surface lime salts, low soil organic matter, warm temperatures and dry windy conditions.
To reduce volatilization loss, ensure proper fertilizer placement into the soil, broadcast just before a rain, use a urease inhibitor such as Agrotain, or use slow-release fertilizers like ESN (environmentally smart nitrogen) urea. Product choice is also a factor. Ammonium sulphate would have very little volatilization, and would be preferred in a situation where incorporation won’t take place.
Weeds
Weeds can contribute to poor nitrogen fertilizer efficiency by competing with crops for uptake. The competitive ability of the crop for fertilizer uptake can be improved by banding rather than broadcasting fertilizer (so fertilizer is placed near crop roots) and by timing the fertilizer applications when it will favour crop uptake rather than uptake by weeds.
Erosion
Erosion of topsoil carries significant nitrogen and other nutrients away from the field. Using soil conservation techniques will help minimize such losses.
Clay particles
Another potential loss mechanism occurs when ammonium is fixed into the crystal structure of certain clays. Some soils contain expanding type clays that allow ammonium to enter within the plates of the crystal structure and become ‘fixed.’ This ammonium is trapped within the crystal lattice is held tightly and unavailable for root uptake.
Canola response to fertilizer nitrogen
Canola needs 2.5 to 3.5 pounds per acre of available nitrogen per bushel of seed yield. A canola crop yielding 50 bushels per acre takes up 125-175 pounds of nitrogen, and of that, 88-105 pounds are removed with the seed.
Some of this will come from inorganic soil reserves and from mineralized of organic matter. Organic matter is an organic nitrogen storehouse, but it needs to be decomposed by soil microbes (through the mineralization process) before becoming available for root uptake. Mineralization of soil organic matter is fairly slow and variable, generating six to 20 pounds of available nitrogen per acre for each percentage point of organic matter. For example, soil with four per cent organic matter will supply 24 to 80 pounds of nitrogen through the growing season. Warm conditions, ample moisture and high microbial activity increase the rate of mineralization. Soil building factors such as reduced tillage, manure applications, high yielding production and return of crop residues can all increase organic matter content and potential mineralization over time.
The rest of the available nitrogen has to come from fertilizer, in the form of chemical fertilizers or organic fertilizers such as manure or compost.
Canola on deficient soils responds very well to nitrogen fertilizer applications. The most obvious response to fertilizer nitrogen is an overall increase in plant growth. Research generally confirms that nitrogen fertilizer increases canola leaf area index, leaf duration, plant weight, growth rates, number of flowering branches, plant height, number of flowers, number and weight of pods and seed yield. Deficient plants are spindly and pale green when compared to those with adequate nitrogen. Therefore, good nitrogen fertility is necessary to produce a large, photosynthetically efficient leaf area that will support high numbers of flowers, pods and seed yield. Several factors impact the canola crop’s response to nitrogen fertilizer.
Moisture
Moisture is a major limiting factor for canola yield potential and must be considered when setting fertilizer rates and budgets. Canola needs 25 millimetres (1 inch) of available moisture through the growing season for every four bushels per acre of yield potential. A 50-bushel crop will need roughly 300 millimetres (12 inches) of moisture.
However, heavy nitrogen fertilization can reduce canola yields when excellent spring moisture conditions are followed by drought. Under this condition, the nitrogen stimulates larger leaves, and increases transpiration and moisture use. As a result, soil moisture can be depleted, leaving little for flowering, pod production and seed fill. Excessive nitrogen fertilization can also reduce yields by promoting lodging, delaying maturity that may increase fall frost damage, and increasing foliar disease (due to the dense canopy and lodging). Selecting an adequate nitrogen fertilization rate for a yield target by taking into account available nitrogen from a soil test is crucial for maximizing production.
Under dry soil conditions, root growth and activity are reduced, resulting in less nitrogen uptake. In addition, soil microbial growth is slower. This reduces nitrogen release from soil organic matter, but also reduces temporary unavailability by soil microbes due to immobilization. Normally, more plant available nitrogen is left in the soil after a dry growing season than after wet seasons.
Yield
Canola yield potential strikes a balance between available nutrient and growing season moisture and temperature. Growers in a climate zone where canola can yield 60 bushel per acre will apply higher rates of fertilizer than growers in a climate where canola yields typically reach 40 bushel per acre, for example. If a grower consistently achieves or surpasses their yield target, adjusting it higher and fertilizing accordingly will improve overall profitability.
The amount of nitrogen a crop will use also depends on the yield potential of the genetics selected for planting. Hybrid canola cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More generally have a higher yield potential than open-pollinated cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More grown under similar environmental conditions 2, 3, 4. As such, they utilize more nitrogen to attain their optimum yield. A 2009 study concluded that the nitrogen requirement for hybrid canola was 50 to 60 kilograms of nitrogen per hectare (45-54 pounds of nitrogen per acre) greater than for open-pollinated canola 4. This is under good growing conditions when canola has the opportunity to reach its full yield potential.
Quality
Nitrogen fertilization, especially at higher rates, may slightly decrease the canola seed oil content. Plant breeders report that oil and protein contents are often inversely related, so attempts to change one causes an opposite change in the other component. However, while the oil content percentage in each seed may decrease at high nitrogen rates, the total oil yield per acre still increases because yield increases more than oil level decreases.
Higher amounts of available nitrogen later in the growing season can increase protein in the seed. However, when nitrogen fertilizer is added under conditions of sulphur deficiency, there may be an increase in free amino acids due to hampered protein synthesis rather than a rise in protein. Additional nitrogen should not be added to a canola crop that is deficient in sulphur, as this will worsen the sulphur deficiency.
Ample to excessive nitrogen availability can also extend crop maturity. This increases the risk of a fall frost preventing the plant from reaching full maturity, which can subsequently result in smaller seeds and higher seed chlorophyll content, both which reduce quality. Excess nitrogen placed too close to the seed row may delay emergence or decrease stand density, which will also delay maturity.
Economics
When it comes to crop nutrition, the biggest return on investment comes from nitrogen. Full economic value of high-yielding canola cultivarsCultivars are variants in a species developed through the intervention of humans (despite the term 'variety' often being incorrectly used to describe this). Cultivars can be open-pollinated type, hybrid, synthetic, composite, etc. More is realized when fertilizer rates are at or above the current recommended rates.
Fate of applied nitrogen fertilizer
Research in western Canada has found that nitrogen fertilizer use efficiency (fertilizer nitrogen recovered in seed) rarely exceeds 50 per cent. The remaining 50 per cent stays in plant parts other than the seed (and eventually returns to the soil), and a significant portion remains in the soil as mineral or organic nitrogen, to be recovered by subsequent crops. In the Prairies, a relatively small amount of the nitrogen seems to actually be “lost” in terms of irrecoverably gone via leaching, denitrification or volatilization.
Identifying nitrogen deficiencies
Nitrogen deficiency symptoms include:
- Leaves at the bottom of the plant are yellowing prematurely.
- Plants have reduced biomass (appearing thin and spindly).
Healthy canola plants with adequate nitrogen have dark green leaves. Nitrogen is mobile within the plant and can be moved from older to younger leaves and pods. Therefore, nitrogen deficiency symptoms first show up in older leaves as pale green to yellow colouring. These older leaves tend to die early, turn brown and drop off prematurely. Overall plant growth is slow, with short thin stems, small leaves, and few branches. The amount and time of flowering is restricted, and pod numbers are low.
In healthy canola, plant tissue tests of above ground material at flowering will show more than 2.5 per cent nitrogen.
Proper diagnosis of nutrient deficiency should use most of the following tools:
- Soil test. Notice if any obvious shortages are evident. Other soil test parameters such as texture, pH and electrical conductivity may also provide clues in the diagnosis. Consider soil quality variation within the field. For example, since nitrogen is soil mobile, sandy areas may show symptoms earlier or be more obvious.
- Fertilizer history. Consider what rates and source products have been applied on that field in past years. Include crop yields and consider whether rates have been adequate to match removal.
- Tissue test. Do not use this alone. A tissue test may show that the plant is deficient but this could be temporary due to environmental stresses, for example. Nitrogen may be in the soil at adequate rates, but the plant simply can’t access it. So even if the tissue test shows deficiency, top dressing may not be the answer. Plants may recover without top dressing once the environmental stress subsides. When tissue testing, compare healthy areas to poor areas of the field and look for differences.
- Herbicide history. Consider if there any chance of carryover from products applied one or two years ago. In dry conditions or very wet conditions, herbicides can take longer than expected to break down to safe recropping levels for canola and either restrict root growth or cause symptoms that could be mistaken for nutrient deficiencies.
- Environment. Cold, wet, hot and dry can all stress canola plants, creating symptoms that may look like nutrient deficiencies. If most of your canola fields in the area are affected and neighbours’ fields have similar symptoms, the cause is more likely to be environmental — frost, excess moisture, etc.
- History of the land. Recently broken forage land is likely to be depleted in a lot of nutrients. Conversely, land that has had maximum rates of manure application or heavy rates of inorganic fertilizer in recent years may be less likely to exhibit deficiencies, making other causes more likely, especially if environment had kept yields below target levels.
- Look at other fields for similar symptoms. When diagnosing for a specific nutrient, target the crop that tends to be most sensitive to that nutrient. If your farm is depleted of copper, for example, this deficiency is likely to show up in wheat before any other crop.
Placement of fertilizer
Fertilizer placement in the soil, as opposed to on the surface, greatly minimizes losses from volatilization and immobilization and enhances overall nitrogen fertilizer recovery. The main options for fertilizer placement include seed row, band, broadcast and top dressing.
Seed row
Canola is sensitive to nitrogen placed in the seed row. Canola seedlings are injured by excessive seed row nitrogen by the salt effect that reduces water uptake by the seed, and by ammonia toxicity.
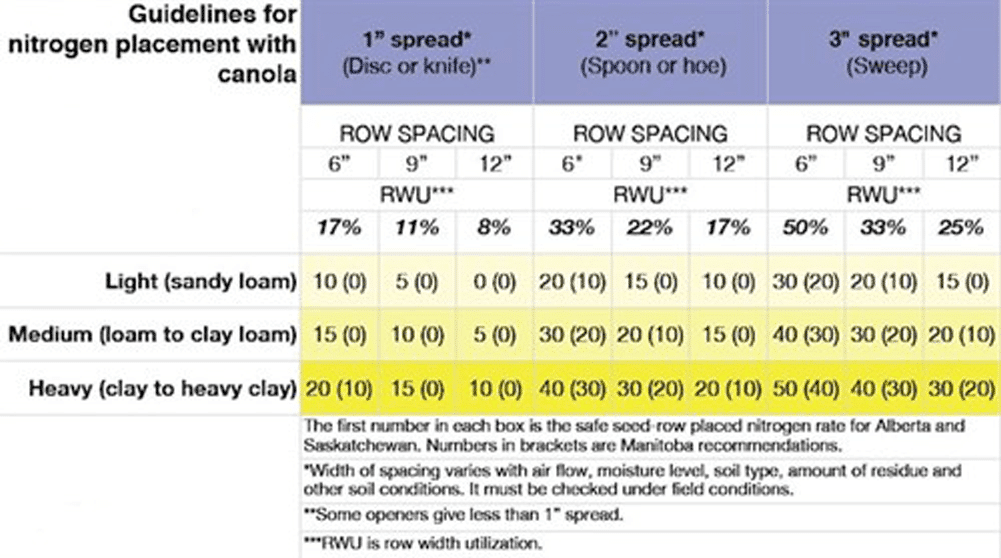
Numerous trials have examined the safe seed row nitrogen amounts with various openers and configurations. Research conducted in Alberta from 1992-96 illustrates the effect of seed row nitrogen on canola emergence and yield. The research involved 32 site years with canola at various locations. The highest nitrogen rate and least seedbed utilization (SBU) reduced canola emergence 90 per cent of the time and reduced yield 45 per cent of the time. Sites with limited moisture (due to sandy texture, low seedbed moisture or dry conditions) experienced the greatest reduction in emergence and yield two weeks after seeding, with 101 kilograms of nitrogen per hectare (90 pounds of nitrogen per acre) as urea and low seedbed utilization.
The safe rates of seed-placed nitrogen table shows the approximate safe rates of seed row granular nitrogen fertilizer based on Prairie research to date. In canola, there is no significant difference in seed row safety between urea (46-0-0), ammonium sulphate (21-0-0-24) or ammonium nitrate (34-0-0). However, anhydrous ammonia (82-0-0) must be placed separately from the seed. If moisture conditions are dry, the safe amount of seed row nitrogen should be reduced by half. The safe nitrogen rates are in addition to nitrogen contained in safe rates of seed-row phosphate fertilizer.
Putting down seed and all the planned fertilizer in one pass is a common practice on the Prairies. But for canola, most of that fertilizer has to go in a side band or mid-row band. The table shows safe rates of seed-placed urea, given in pounds of actual nitrogen per acre. (Multiply by 0.46 to get pounds of urea.)
Manitoba has lower safe rates than Saskatchewan and Alberta. This distinction is based on soil characteristics. Manitoba soils tend to have high soil pH, and high levels of free lime or carbonates. Under these conditions, urea tends to release more of its nitrogen in the free ammonia form (NH3), which is more damaging than the ammonium form (NH4+).
When selecting seed-placed nitrogen rates, note that the suggested rates (in the table) assume that the grower is already applying a safe rate of seed-placed monoammonium phosphate (MAP). MAP is usually safe at rates below 45 kilograms per hectare (40 pounds per acre) of product. Some growers also use diammonium phosphate (DAP) for starter phosphorus, but this form has a higher nitrogen component. Safe rates of seed-placed nitrogen will be lower if the grower is using DAP instead of MAP.
Factors that affect safety of seed-placed nitrogen are:
Soil texture: The lighter the soil texture, the higher the risk to emergence damage and yield loss. Sandier soils are more risky than clays. Clay soils hold more nitrogen in the ammonium (NH4+) form. In sandy soils, more ammonium converts to ammonia (NH3), which is the form that causes seed and seedling damage because it is more toxic to plant tissues.
Seedbed moisture conditions at seeding: With moist soil conditions, water dilutes the concentration of nitrogen molecules around the seed and seedling. Water also disperses nitrogen molecules throughout the soil, reducing concentrations around the seed. In dry conditions, seed-placed nitrogen fertilizer tends to produce higher concentrations of ammonia and ammonium that can damage young seedlings. Seed-placed nitrogen rates should be lowered when conditions are drier than normal.
Fertilizer source: Ammonia can damage crops through direct toxicity while nitrate will damage seedlings by desiccation through the salt effect. Canola is sensitive to both the salt effect and direct toxicity, which is why there is no distinction made between products containing straight ammonium and those containing a mixture of ammonium and nitrate nitrogen when it comes to safe seed-placed nitrogen rates for canola. Nitrogen products with a polymer coating (e.g. ESN) can allow for higher safe-rates of seed-placed nitrogen by lowering the concentration of ammonia that is in contact with the seedling. Anhydrous ammonia should never be placed in the seed row.
Row space: Wider rows increase the risk of seedling damage, assuming that fertilizer rates per hectare (or acre) stay the same. The same amount of fertilizer directed into fewer rows (due to wider spacing) increases the concentration of fertilizer in each seed row.
Application rate: The higher the nitrogen rate, the higher the risk of emergence damage and yield loss resulting from lower plant counts.
Crop type: Generally, small seeded crops such as canola are more sensitive to seed-placed nitrogen. Do not use the same rates for canola as for wheat, which is less sensitive to ammonia toxicity and salt effect.
Soil pH: Safe rates are lower in high pH soils. At higher pH, more of the nitrogen from urea is in the free ammonia form (NH3) vs. ammonium (NH4+). Ammonia is more damaging.
Band
Band placement away from the seed row can be used to avoid toxicity and to improve fertilizer use efficiency. Banded nitrogen fertilizer is usually more efficient than broadcast incorporation because concentrating fertilizer in the band reduces the contact with soil and microbes. This slows the conversion of ammonium to nitrate, reducing the risk of denitrification and leaching. Placing fertilizer below the crop residue also reduces the risk of immobilization. The banding benefit varies between soils and years mainly due to differences in moisture and susceptibility to loss.
Pre-plant banding can be difficult logistically in the spring, and may do excessive damage to the canola seedbed. Fall banding on relatively well-drained soils after the soil has cooled can spread the workload without significantly lowering nitrogen efficiency, and often allows growers to buy fertilizer at lower cost than in the spring. The banding depth often is eight to 10 centimetres (three to four inches). Under dry or sandy soil conditions, ensure anhydrous ammonia is placed deep enough to prevent visible gaseous loss, and ensure the soil flows well around the openers to permit a good seal behind the shanks. Spring banding can be shallower due to better moisture and tilth. However, in dry springs the banding operation can reduce seedbed moisture and quality.
Spring banded anhydrous ammonia can be immediately followed by seeding, providing there are several inches of vertical separation between the injection point and seed depth. Canola emergence directly over the bands may be slightly reduced, but yields are generally not affected.
Research conducted at the Agriculture and Agri-Food CanadaAgriculture and Agri-Food Canada is a department of the Government of Canada. More Scott, SK Research Centre on the safety of seeding directly after banding anhydrous ammonia 10 to 15 centimetres (four to six inches) deep. Yields varied between seeding dates following anhydrous banding over the years with no relationship between yields and plant stands. Proper soil packing over the seed row (to firm the soil disrupted by the banding operation) was deemed more important to avoid stand reduction than was potential injury from the banded ammonia. No difference was found between banding ammonia parallel to and perpendicular to the seed row.
Side banding involves placing the fertilizer band to the side and often below the seed during the seeding operation. This method has good nitrogen use efficiency and avoids seed row toxicity if the separation is maintained under field conditions. However, side banding at seeding does involve more complicated, costly openers, increased draught and wear. Openers should be properly maintained to ensure adequate separation of the seed and fertilizer is maintained.
Mid-row banding involves placing fertilizer between every second seed row during the seeding operation. This method also has good nitrogen use efficiency and avoids seed row toxicity. Due to the distance between the mid-row band and the seed band, high rates of nitrogen can be very safely applied in a range of conditions.
Paired row banding involves placing fertilizer between two rows of seed, all from the same opener, in a one pass seeding operation. The seed and fertilizer are usually on the same plane, so care should be exercised to ensure adequate separation between these bands.
In recent years, openers have been designed that allow anhydrous ammonia to be side-row or mid-row banded during seeding. Ensure the anhydrous ammonia is horizontally separated from the seed by at least 5 centimetres(2″).
Broadcast
Dry or liquid nitrogen fertilizer can be applied (or broadcast) to the soil surface, and then incorporated into the soil with a tillage implement. Although this method can save on time and labour, fertilizer efficiency is usually sacrificed. Immobilization will be higher with broadcast nitrogen applications relative to banding because it will be placed in higher amounts of crop residue. Under dry conditions, broadcast-incorporated fertilizer can be stranded in dry surface soil, making it not accessible by plant roots growing down into moisture. Broadcast applications can have relatively good efficiency if a good rain follows immediately afterward. However in wet conditions, broadcast-incorporated fertilizer is more vulnerable to losses due to denitrification and leaching than banded fertilizer. Use of a urease inhibitor such as Agrotain can reduce these losses. Broadcasting after crop emergence or top dressing generally has lower efficiency, but it can serve as a rescue treatment when poor fertility leads to plant deficiencies following emergence, or when improved environmental conditions require a reassessment of initial yield targets.
Top dressing
Considerations
A nitrogen top up can make sense (economically) for canola if:
- Growing conditions improve after seeding. If conditions were too wet or too dry at the time of seeding, growers may have cut back fertilizer rates in response to lower yield projections. If conditions improve in June and a good stand emerges, growers may see a yield benefit from a nitrogen top up. In dry conditions, applying at least 66 per cent of the recommended nitrogen rate at seeding then consider topping up if conditions improve, as a risk management option.
- Saturated soils impede good seed placement. This expands on the previous point. When the only choices to get canola seeded are mudding inSeeding when it is too wet (and there are some muddy conditions). In these instances the field may support the tractor and drill, but the soil will not fill in properly over the furrow and the packers may get muddy and slide. More or broadcasting, cutting back on nitrogen rates at seeding may be a good practice to reduce nutrient losses and to allow for stand establishment assessment. If the crop becomes well established, an investment in more nitrogen fertilizer would be warranted.
- High nitrogen losses are likely to have occurred. Wet soil conditions can accelerate nitrogen losses through leaching and denitrification. Fields may need a nitrogen top up to reach their yield potential, but make sure a sufficient canola stand survived the wet conditions before investing in a fertilizer top up.
- The crop is showing signs of nitrogen deficiency. Nitrogen deficiency symptoms first show up in older leaves as pale green to yellow colouring, and sometimes purpling. Tissue analysis may confirm these observations, but be sure to follow lab rules for sampling. Turnaround time is another hurdle. Results may not come back in time to take action. As an alternative, growers or agronomists could do a small experiment by spreading fertilizer on the surface and watering it in to see how plants respond. A small patch could be done by hand. If the plants in the patch green up, this suggests a nitrogen deficiency.
- A grower cannot efficiently place all the fertilizer needed through the seeding tool. Some growers will address this with a top dress application of nitrogen after crop emergence.
- In cases of poor root growth. Plants may be able to reach leached nitrogen later in the season as their root systems fully develop, but canola in fields with excess moisture may not develop the root system to reach that far. If roots have been growing to the side and there is no dominant tap root, then the tap root is unlikely to develop fully. Lateral roots may start to turn downward as the top layer dries, but they may not have the reach of a good tap root. In this case, a top dress of nitrogen may help the crop — as long as good growing conditions have returned, to improve the potential return on that nitrogen investment.
Timing (plant stage)
Ideally, a nitrogen top dress should occur before the four to six-leaf stage of the crop. For canola, maximum nitrogen uptake is from the start of flowering to the end of pod formation. Application by the four to six-leaf stage gives time for rainfall to move fertilizer into the root zone, making it available before the peak uptake period begins. Top ups can be effective after the four to six-leaf stage (if weather prevents earlier application), assuming that soil nitrogen levels are sufficient to carry a crop through the first few weeks of peak growth. In that case, a later top up may provide the nitrogen necessary to carry the crop through to full yield potential after original reserves are drawn down.
Source
Nitrogen source options for in-crop top up applications are urea (dry), urea ammonium nitrate or UAN (liquid) or ammonium sulphate, if sulphur shortages are also expected. UAN dribbled on the surface is less prone to losses than dry urea broadcast on the surface. But both surface applications require rain soon after application to move the fertilizer into the soil, both to limit volatilization losses and to make the nitrogen available to the plant roots. The key for UAN is that it reaches the soil surface. UAN applied in no-tillA type of tillage management technique where soil is undisturbed from harvest through planting, seeding with narrow knives/hoes or discs. More situations with high residue cover can be absorbed by the residue and become unavailable to the crop. Surface dribble bands are required for this option. Spray applications (flat fan nozzles) are less effective and will damage leaf tissue.
Ammonium sulphate is less volatile than urea and will not lose nitrogen as quickly when surface applied, except under high pH carbonated conditions. Agrotain or other products containing a urease inhibitor will reduce losses for surface applied urea and UAN. ESN is not recommended for post seeding applications because its slow-release feature may not release the nitrogen in time. If using granular, apply when leaves are dry to make sure prillsSmall, round pellets of dry fertilizer. More roll off onto the ground and don’t cause leaf burn.
If using liquid, apply when leaves are moist from early dew or a light rain so liquid nitrogen fertilizer runs off quickly. Applying when hot and dry can increase absorption of liquid into the plant, increasing the amount of leaf burn. Consider adding some extra water to the tank in these conditions if waiting is not an option. Use straight stream applicators, not fan nozzles, and use some pressure to drive the stream to the ground. Keep in mind that windy conditions may disrupt the streams and lead to increased plant coverage and potential for damaging greater leaf area.
Rates
Even a small amount – 20 pounds of (actual) nitrogen per acre, for example – can provide an economic return if crops are deficient and conditions are such that the plant can access the nitrogen. Some growers will apply higher rates, but it should be noted that a nitrogen top up will extend canola’s vegetative period and delay maturity. Consider the calendar date and the fall frost risk when making nitrogen top up decisions.
Growers may need to top dress up to 30 pounds per acre of actual nitrogen to visually notice an improvement, but yield differences for rates lower than 30 pounds per acre may show up at harvest.
Placement (method)
Broadcast spreading of urea or surface dribble banding of UAN are the most common and fastest methods, but these methods can also result in the highest losses if rain doesn’t follow soon after the application. (Although losses with UAN tend to be lower than with urea.) Urease inhibitors like Agrotain can help to minimize these losses.
Fertilizer placement in the soil, as opposed to on the surface, reduces losses from volatilization and immobilization and enhances overall nitrogen fertilizer recovery. This may be difficult to achieve in practice because spoke wheel applicators are currently hard to find. Side dressing is an application method that can be used to band the nitrogen in the soil rather than leaving it on the surface. Side dressing utilizes more specialized equipment than top dressing.
Tank mixing liquid nitrogen with herbicide and applying through the sprayer tank provides only a few pounds of actual nitrogen per acre. This practice also applies fertilizer to the leaves instead of the soil surface. Fertilizer applied to leaves can damage the crop, and the crop can’t take up much nutrient through the leaves. Most of the uptake will occur through the roots once rain washes the fertilizer off the plants and into the soil.
A variable rate applicator using optical sensors (e.g. GreenSeeker) is a high tech option. Applicators with optical sensors estimate crop biomass, yield potential and crop nutrient requirements based on reflectance of certain wavelengths of light emitted from the sensor and then reflected back to it. The sensors can be attached to a nitrogen applicator and provide continuous and instantaneous readings for variable rate applications.
Economics
Whether top dressing makes sense financially may depend on the conditions of the crop and whether the crop is deficient in nitrogen. If rates were more than adequate for a high-yielding crop, some nitrogen could be lost to leaching or denitrification and not hurt yield potential too much. The healthy crop may also access leached nitrogen lower in the soil profile later in the season. But if nitrogen application rates were reduced in response to rising costs or lower yield targets at seeding time and the crop is looking better than expected, this crop might benefit more from a top up.
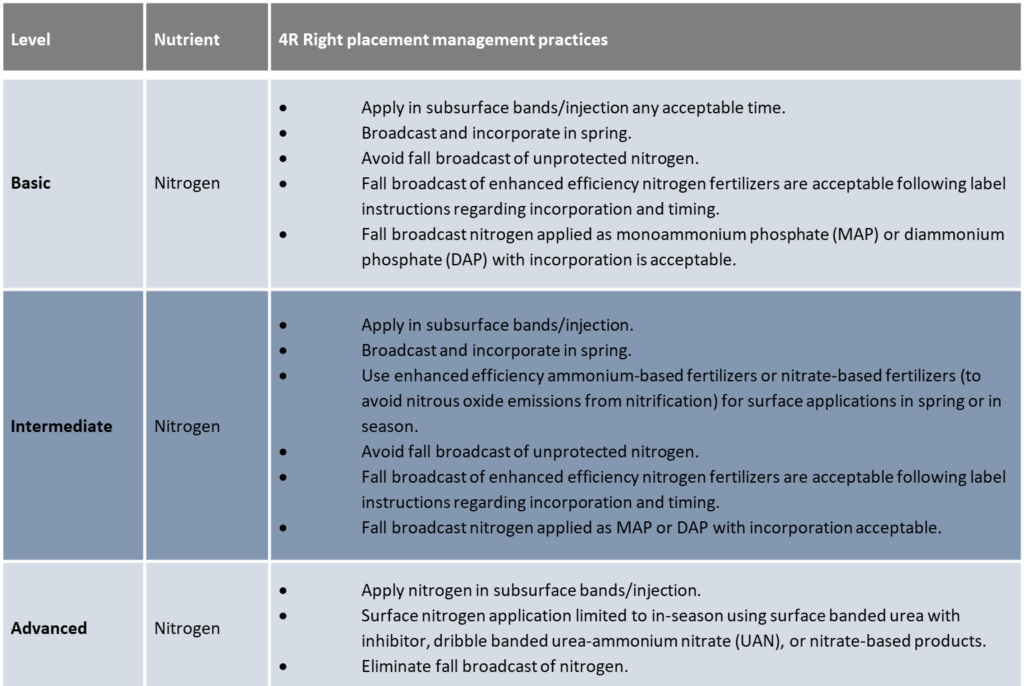
4R Practices for spring cereal, oilseed, and pulse rotations in the Canadian Prairies without manure or compost – Prairie and Boreal plains ecozones
Timing of application
Nitrogen fertilizer efficiency is greatly affected by the placement method and the application date. However, differences in crop response between placement methods or timings vary widely between years or fields due to variability in weather, soil type and drainage.
Fall
Nitrogen fertilizer banded too early in the fall increases the potential for losses on wet, poorly drained soils. Delay banding until soil temperatures have cooled to less than 10 degrees Celsius on well drained sites, and less than 5 degrees Celsius on poorly drained sites. This will significantly reduce losses by reducing the rate of change from ammonium to the more vulnerable nitrate form. Unfortunately, the opportunity to fall band fertilizer into cold soil is quite short since snow or frozen soil can occur without much warning. On average, fall soil temperatures on the prairies drop one degree Celsius every five days. In the Black and Gray Wooded soil zones, fall soil temperatures usually decline to 10 degrees Celsius by the last week of September. In these zones, since the banding benefit is significant due to typically wet spring conditions and moderately high nitrogen rates, it may be best to begin fall banding in late September on well-drained soils. This is earlier than the normal practice.
Spring
Generally, nitrogen fertilizer applied at or near seeding is the most efficient. The major disadvantages of applying all the fertilizer nitrogen at seeding are:
• increased time required to seed and fertilize during the short seeding season
• general trend of higher fertilizer prices during the spring season compared to fall
• risk of reduced seedbed quality due to extra tillage or soil disturbance at or near seeding
• seed safety nitrogen limits
• fertilizer applicators being unavailable during the busy spring season
| Table 5. Relative efficiencies of fertilizer timing and placements under different moisture scenarios |
Data source: Ridley (1975) 5
More recent work by Tiessen et al. showed that later dates and lower soil temperatures increased crop nitrogen use efficiency of fall-banded urea (relative to spring-banded urea), in low landscape positions. In the high landscape positions, the performance of fall-banded urea was not related to any measures of time and/or soil temperature 6.
Product choices
Available forms
There are five available forms to consider for nitrogen fertilizer applications: anhydrous ammonia, ammonium sulphate, area, urea ammonium nitrate and manure.
Anhydrous ammonia
Anhydrous ammonia (82-0-0) is delivered as a liquid in pressurized tanks, and converts to gas at atmospheric pressure. When it is injected into the soil, an individual NH3 molecule reacts with a water molecule in the soil to form an ammonium and a hydroxyl ion (NH3 + H2O → NH4+ and OH–). This results in the formation of a zone of a high pH solution of ammonium hydroxide localized around the point of injection. The ammonium (NH4+) ions will at first be adsorbed onto the negatively-charged soil colloids but will begin to spread out slowly due to chemical diffusion. The oxidizing bacteria present in or near the zone of application will cause nitrification of ammonium (NH4+) to nitrate (NO3–). The mobile nitrate (NO3–) ions will then flow with water in the soil and spread out into the soil and be intercepted by crop roots. It takes time for nitrification to occur because it is a microbial process. The nitrifying bacteria need adequate moisture and temperature to function. If anhydrous ammonia (NH3) is applied when soil temperatures are cool and soil moisture is limited, the spreading out of mobile nitrate (NO3–) from the zones of injection may be delayed. This is sometimes observed as striping in fields, with dark green crop where the fertilizer was applied and yellowing crop in the deficient soils between each injection zone. This usually clears up with rain.
Denitrification and leaching losses from anhydrous ammonia applications are usually low unless it is applied very early during a warm long fall. Nitrification of most of the ammonium (NH4+) may occur, and if waterlogged conditions are experienced the following spring, denitrification of nitrate (NO3–) may be a problem. If anhydrous ammonia is applied later in the fall when soil temperatures are reduced below 10 degrees Celsius, or in the spring, denitrification losses are normally small.
Ammonium sulphate
Ammonium sulphate (21-0-0-24) is not subject to denitrification losses in the short term, and is well-suited for surface applications due to low or no ammonia volatilization losses, except on high pH or carbonated soils. It is also an excellent sulphur fertilizer source. Its disadvantage is a lower nitrogen analysis than other nitrogen sources, resulting in higher transportation costs.
Urea
Urea (46-0-0) has the highest analysis of nitrogen of all granular fertilizers. It is hydrolyzed in the presence of the naturally occurring urease enzymes in the soil or on crop residues to release plant available nitrogen. A rainfall of 12-20 millimetres (half to three quarters of an inch) is usually sufficient to dissolve and move the soluble urea into the soil before losses occur. If incorporated or banded in the soil, volatile losses are minimal.
UAN
Urea ammonium nitrate, or UAN (28-0-0), is a liquid fertilizer option. It is a mixture of dissolved ammonium nitrate and urea in an approximate 50:50 blend along with enough water to keep it in solution. It has loss properties that are characteristic of a blend of urea and ammonium nitrate. Dribble banding is recommended for surface applications, because if sprayed evenly over the surface of the soil into a thatch cover, much of the fertilizer may adhere to the surface residue and not move readily into the soil beneath the residue. It will be immobilized in this residue (e.g. established forage crop or a no-tillA type of tillage management technique where soil is undisturbed from harvest through planting, seeding with narrow knives/hoes or discs. More seeded crop). If banded into the soil, it will perform similarly to banded urea or banded ammonia in the spring.
In the fall, it will be more subject to loss than the ammonium or ammonium-producing fertilizers because a greater proportion is initially in the nitrate form. There is some mention that this form of nitrogen fertilizer is more available to plants because it comes pre-dissolved in water. However, this is a misconception because the amount of water in the fertilizer is minuscule compared to the amount of water present in a soil near normal field capacity.
Manure
Manure can be a very good source of nitrogen, but manure should be tested for nutrient content per tonne before setting application rates per hectare or acre.
Enhanced efficiency fertilizers
Grant, Malhi and Schoenau describe urease inhibitor, controlled release nitrogen and nitrification inhibitor products that can enhance fertilizer efficiency in their publication 7.
Urease inhibitors
Urease inhibitors can decrease ammonia volatilization from surface applications and seedling damage from seed-placed ammonium or ammonium-producing N fertilizer. This could help growers reduce soil disturbance and application costs.
Urea will not volatilize or cause seedling damage until after it has been hydrolyzed to ammonium in a reaction catalyzed by the urease enzyme. Urease inhibitors slow the rate of hydrolysis to reduce the concentration of ammonium and ammonia present in the soil solution. This allows time for rainfall to move urea into the soil where the released ammonia will be less subject to volatilization, or for the urea to move away from the germinating seedling, reducing the risk of seedling damage.
Use of urease inhibitors with fall-banded urea may slow the release of ammonia and its subsequent conversion to nitrate, reducing the risk of nitrate leaching or denitrification.
Urease inhibitors are most likely to increase crop yield where yield potential is high, soil N levels are low, and soil and environmental conditions promote extensive volatilization. Potential volatilization, and hence potential benefits from the use of urease inhibitors, will be higher where: incorporation is difficult; where there is little opportunity for urea to move into the soil with infiltrating water; or where the soil has a high urease activity because of lack of cultivation or the accumulation of organic material. The risk of volatilization and seedling damage is higher on high pH, calcareous soils.
Controlled release nitrogen
The product currently available is polymer-coated urea, ESN, which releases urea fertilizer into the soil solution at a rate limited by moisture and controlled by soil temperature. Controlled release urea provides its greatest benefit under warm, moist conditions that promote high loss. The potential benefits will also increase if the fertilizer is in the soil for a longer period of time before crop uptake.
If conditions are dry and losses of conventional urea are low, controlled release urea may not increase yield compared to in-soil banded uncoated urea. Under wet conditions, controlled release urea should reduce the risk of leaching and/or denitrification by more closely matching uptake with supply, hence reducing losses.
Controlled release urea can also be used to reduce seedling toxicity. With ESN, rate of release is controlled by soil temperature, with release increasing as the soil temperature increases. This keeps the concentration of ammonia and ammonium lower early in the season when seedlings are most sensitive and releases the nitrogen in greater amounts as plant growth increases later in the season. An Agriculture and Agri-Food CanadaAgriculture and Agri-Food Canada is a department of the Government of Canada. More study found that using ESN for seed-row placed urea actually increased seed safety more than increasing the seed row width from 1.9 centimetres (three quarters of an inch) to 7.6 centimetres (three inches).
Nitrification inhibitors
These products slow nitrification of ammonium-producing fertilizers by interfering with the action of Nitrosomonas bacteria. Slowing nitrification allows the fertilizer to maintain the ammonium form longer, reducing the concentration of NO3- in the soil solution and thus reducing the risk of leaching, denitrification, and N2O emission release.
Both agronomic and environmental benefits of nitrification inhibition will be greatest where potential losses through leaching and denitrification
are high, for example under wet soil conditions. Benefits are unlikely in dry or well-drained soils, since leaching and denitrification losses are limited by a lack of moisture.
Footnotes
- Karamanos, R.E. 1979. Variations in natural N Abundance as an index of past nitrogen cycle stresses. Ph.D. Thesis. Saskatoon: University of Saskatchewan, 165.[↩]
- Harker, K. N., O’Donovan, J. T., Turkington, T. K., Blackshaw, R. E., Lupwayi, N. Z., Smith, E. G., Klein-Gebbinck, H., Dosdall, L. M., Hall, L. M., Willenborg, C. J., Kutcher, H. R., Malhi, S. S., Vera, C. L., Gan, Y., Lafond, G. P., May, W. E., Grant, C. A. & McLaren, D. L. 2012. High-yield no-tillA type of tillage management technique where soil is undisturbed from harvest through planting, seeding with narrow knives/hoes or discs. More canola production on the Canadian prairies. Can. J. Plant Sci., 92, 221–233.[↩]
- Blackshaw, R.E., Hao, X., Brandt, R.N., Clayton, G.W., Harker, K.N., O’Donovan, J.T., Johnson, E.N. & Vera, C.L. 2011. Canola Response to ESN and Urea in a Four‐Year No‐Till Cropping System. Agron. J., 103, 92-99. https://doi.org/10.2134/agronj2010.0299[↩]
- Smith, E. G., Upadhyay, B. H., Favret, M. L. & Karamanos, R. E. 2010. Fertilizer response for hybrid and open-pollinated canola and economic optimal nutrient levels. Can. J. Plant Sci., 90, 305-310. https://doi.org/10.4141/CJPS09027[↩][↩]
- Ridley, A.O. 1975. Effect of nitrogen fertilizers, time and method of placement on yields of barley. In Proceedings of 19th Annual Manitoba Soil Science Society Meetings. Winnipeg. 1975. pp. 102-111.[↩]
- Tiessen, K.H.D., Flaten, D.N., Bullock, P.R., Grant, C.A., Karamanos, R.E., Burton, D.L., & Entz, M.H. 2008. Interactive Effects of Landscape Position and Time of Application on the Response of Spring Wheat to Fall-Banded Urea. Agron. J., 100: 557-563. https://doi.org/10.2134/agronj2006.0225[↩]
- C. Grant, S. Malhi and J.J. Schoenau, 2010. Improving Nutrient Use Efficiency With Enhanced Efficiency Fertilizers in the Northern Great Plains of North America. In S.S. Malhi, Y. Gan, J.J. Schoenau, R. Lemke and M. Liebig (eds). Recent Trends in Soil Science and Agronomy Research in the Northern Great Plains of North America, 73-94. Kerala: Research Signpost.[↩]