Adequate plant nutrition is important for canola production, which in turn is affected by soil fertility management. The health of the plant will also affect the crop response to stress factors such as disease and adverse weather. Balanced, effective fertilizer management not only contributes to profitable canola yield but also helps to maintain the productivity of the soil.
Plants depend on light, air, water, nutrients, and physical support for normal growth. Soil plays an important role in all these factors except for light. If any of these basic factors are limiting, plant growth will be reduced or the life cycle may not be completed. In other words, plant growth potential is limited by the factor that is in in shortest supply (the principle of limiting factors).
Other factors such as improper management or pest damage can also result in lower yield. Therefore, a systems approach is necessary to integrate all of the controllable agronomic factors in the best combination to achieve the most economic yield.
Essential plant nutrients
Essential plant nutrients describe the elements or compounds needed by plants to grow and complete their life cycle. Essential plant nutrients must be directly involved in some aspect of the plant metabolism such as structural material, enzymes or hormones, and they must not be totally replaceable by another mineral element. Canola requires 14 essential nutrients, which are classified as macronutrients (nitrogen, phosphorus, potassium and sulfur, magnesium and calcium) or micronutrients (iron, manganese, zinc, copper, boron, molybdenum, chlorine and nickel), based on the relative amounts needed by the plant. Macronutrients are needed in large amounts relative to micronutrients. Table 1 shows the relative amounts of nutrients contained in a typical canola crop. Some nutrients can also accumulate at higher levels than what is necessary for growth.
Table 1. Approximate Amounts of Nutrients in the Above- Ground Portion of a 1,960 kg/ha (35 bu/ac) Canola Crop
General nutrient uptake
The level of most nutrients in the plant is much higher than in the water surrounding the roots. For example, typical nitrogen content in a canola plant at the rosette stage would be five to six per cent nitrogen, whereas the nitrogen level in the soil solution of a fertile soil in the spring would contain about 0.0002 per cent nitrogen (in a plant available form on a dry weight basis). Therefore, plant nutrient uptake must be highly selective.
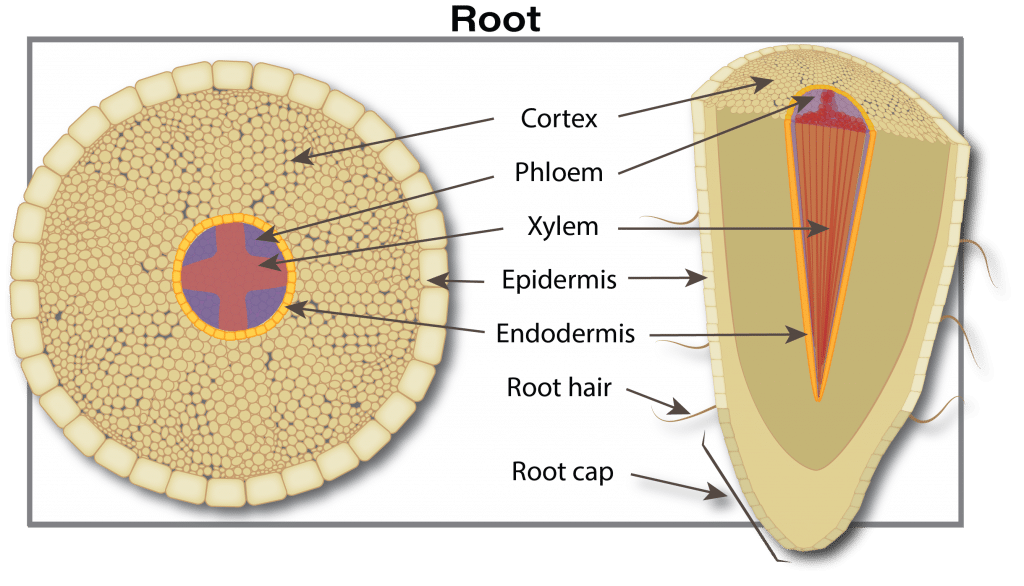
Nutrient uptake begins when plant-available forms move from the soil water through pores in the root skin (exodermis) into the free space of the roots. This free space comprises about five to ten per cent of the root’s internal volume. This movement is a passive process (doesn’t require energy from the plant) driven either by diffusion (movement due to differences in concentration) or mass flow (simply carried by water flowing into the roots). The movement is selective since pores in the free space act as a size filter. Many nutrient ion diameters are much smaller than the pores. For example, potassium and calcium are only 10 to 20 per cent of the pore size, and have easy access to the free space. Large diameter substances such as metal chelates, viruses and fungi are restricted from entry by the small pore size.
As plant roots grow, the soil volume and surface area explored increases, which increases the capacity for nutrient absorption. In addition, roots possess a cationA positively-charged ion (ion with a net positive charge). More exchange capacity (CEC) due to negative charges in cell walls. This negative charge attracts positive ions (cations) like ammonium (NH4+) but repels negative ions (anions) such as nitrate (NO3–). (For details on CEC, see the Soil properties that affect plant nutrition section.)
After entry into the free space, nutrients move into the cell interior by crossing a plasma membrane found on the inside of cell walls. Another similar membrane is found surrounding a large central storage compartment (vacuole) that usually fills more than 80 per cent of the total cell volume. The plasma and vacuole membranes are effective barriers and are the main sites for nutrient uptake selectivity. These membranes contain carrier systems or ion pumps that transport certain nutrients. Such systems require energy from the plant to work, so they are referred to as active uptake systems. This energy demand for ion uptake by roots is considerable, taking up to one third of the energy during rapid growth. The energy for root activity arises from respiration, which requires carbohydrates and oxygen. This explains why nutrient uptake often stops in flooded soils when there is a lack of oxygen.
Some active uptake systems are constant while others have a rate that can be regulated. As the plant level of nutrients and related compounds increases, the root uptake rate can decrease (negative feedback). In contrast, as plants build tissue, the level of nutrient ‘building blocks’ decreases, and the roots are signaled to increase the uptake rate (positive feedback) for nutrients. Passive ion channels through the membranes allow for selective nutrient movement.
The selectivity of the various transport systems across the membranes is not absolute. There is often competition between ions of similar size and charge. For example, chloride (Cl–) competes with nitrate (NO3–). This competition is important in certain saline soils with chloride as a major component of the salt. Most prairie soils contain salt with sulphate as the main anionA negatively-charged ion (ion with a net negative charge). More.
Since cationA positively-charged ion (ion with a net positive charge). More and anionA negatively-charged ion (ion with a net negative charge). More uptake are regulated differently, plants must be able to compensate for differences in electrical charges that arise from disproportionate uptake of cations and anions. Plant cells maintain a pH in the range 7.3 to 7.6 by either releasing or consuming hydrogen cations (H+), which is achieved by formation or removal of organic acids.
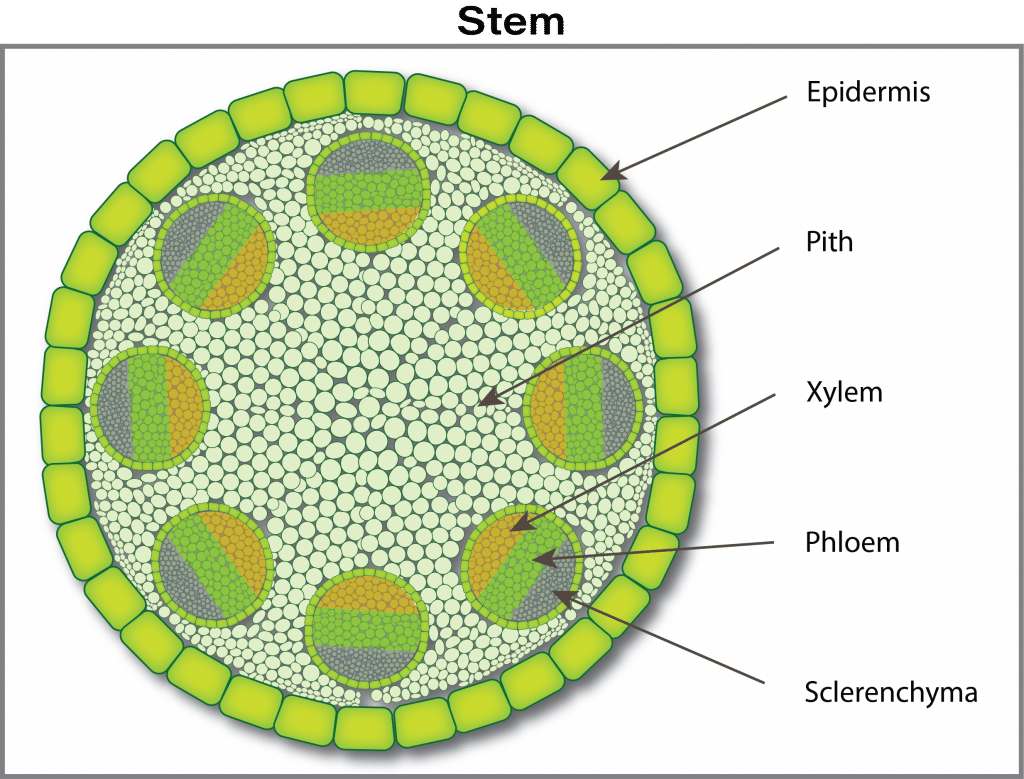
The nutrient journey continues in a path from cell to cell through tiny connecting tubes (plasmodesmata), although some nutrients can continue to move between cells through the free space. The next barrier occurs at the waxy layer (Casparian band) that surrounds the central vascular tissue (phloem and xylem). The phloem and xylem are special tissues that act like highways for nutrient transport from roots to leaves (xylem) and from leaves to growing points and roots (phloem). In young root tips, the Casparian strip is not well formed and thus is an incomplete barrier. The mechanism of how ions pass through the Casparian strip and into the xylem (xylem loading) is not well understood. There is probably a combination of active (ion pumps) and passive channels for ion movement into the xylem.
Xylem loading is regulated separately from root uptake, thus creating a control system for nutrient movement. Nutrients are carried by water up through the xylem. Water flows up through xylem tissues due to a suction-like force created when water evaporates from the leaves, and from slight pressure produced by roots. Once inside the xylem sap, nutrients can be unloaded and reloaded before reaching the end growing points.
Once nutrients reach their targets, there often is considerable recycling, especially for the nutrients that are mobile within the plant such as nitrogen. For example, a normal feature of plants appears to be simultaneous import and export of nutrients from leaves. This dynamic nutrient cycling is termed remobilization or retranslocation. In young vegetative plants, nutrient recycling occurs from the mature leaves to roots and young leaves through the phloem. The remobilization ability of different nutrients affects where deficiency symptoms occur. Deficiency symptoms of plant mobile nutrients such as nitrogen will first appear in old tissues. In contrast, deficiency symptoms of nutrients with limited plant mobility such as sulphur and copper will occur in young tissue, and can hinder flower or seed development.
Nutrient remobilization is particularly important when seeds are forming. At this stage, mobile nutrients are being exported from aging leaves while nutrient imports are decreasing. Also, root activity and nutrient uptake generally decrease by this stage due to drying soils, nutrient depletion in the soil, and a relative shift in the energy supply from roots to developing pods and seeds. Plant parts with a strong energy or nutrient demand are called (nutrient) sinks. As a result, old leaves are sacrificed to supply pod and seed growth. Mobile nutrients in the seed have mostly been transferred from other plant tissue in canola (such as the pods, stems and leaves).
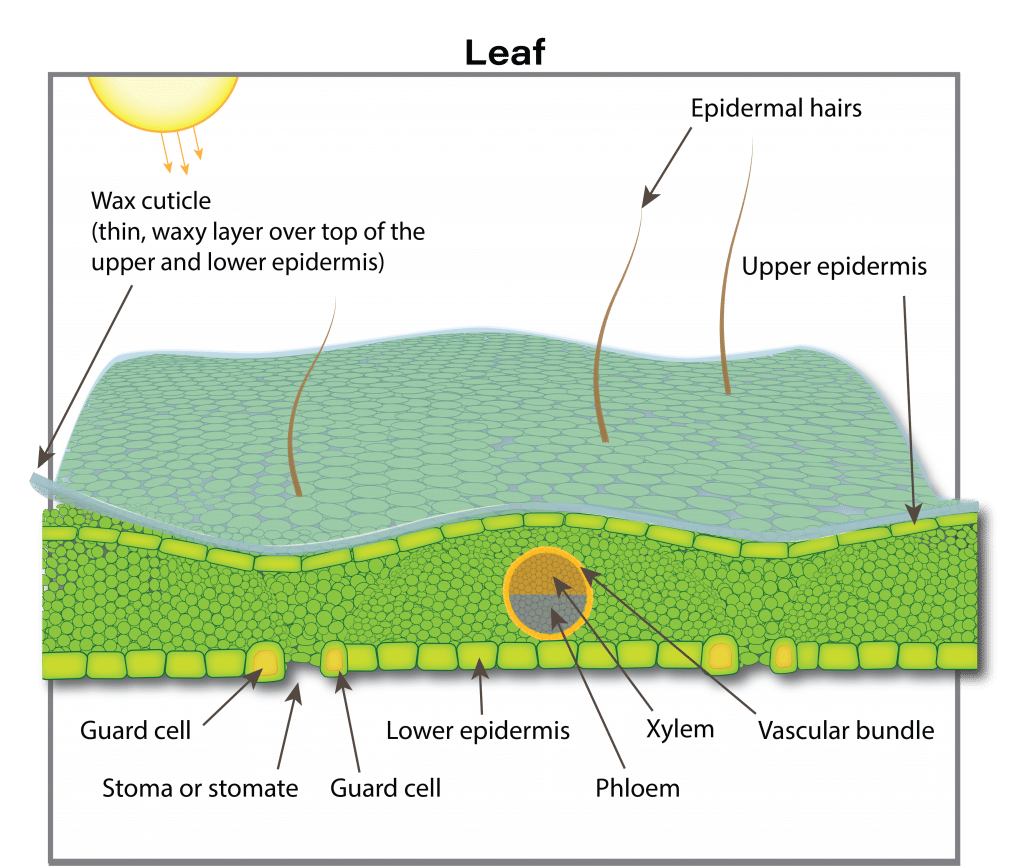
Roots are not the only sites where nutrient uptake can occur. Some nutrients can be absorbed by leaves and other above ground plant parts. Nutrients in a gas form, such as ammonia (NH3), nitrogen dioxide (NO2) and sulphur dioxide (SO2), can enter leaves through leaf pores (stomata) and then be changed into organic forms. These gases can be major air pollution components and in some areas and contribute considerably to plant nutrition. In areas with intensive livestock operations, ammonia uptake can contribute 10 to 20 per cent of the nitrogen for adjacent crops. In a European field experiment, almost half of the total sulphur taken up by rapeseed in the vegetative state came from atmospheric suphur compounds (likely sulphur dioxide, which is readily absorbed by leaves). This may partly explain why sulphur deficiencies have increased in western Canada after environmental regulations enforced cleanup of sulphur emissions from gas plants.
The rate of nutrient uptake by canola varies by nutrient. For nitrogen and sulphur, nutrient uptake is most rapid in earlier growth stages up to the middle flowering stage of the crop. Phosphate uptake is more uniform throughout the growing season. These uptake patterns show the importance of the 4R nutrient stewardship principle of always ensuring the right source of fertilizers are used at the right time, in the right place and at the right rate. For instance, the mid-season plant demand for sulphur illustrates why in-season application of ammonium sulphate can correct a sulphur nutrient deficiency.
Table 2 shows the daily nutrient uptake of several macronutrients at different crop stages.
Table 2. Nutrient uptake (pounds per day) by canola development stage in Melfort, in 1998.
Source: Johnston et al., unpublished data
Soil properties that affect plant nutrition
Soil is a complex mixture of non-living components (minerals, organic matter, gases and liquids) and living organisms (bacteria, fungi, insects, worms, etc.). These factors directly or indirectly influence soil fertility. The solid components make up about half the soil volume, while water and gases make up the other half in the pore space.
Soil mineral particles vary widely in size and are classified by size. For example:
- Rocks (gravel) are larger than two millimetres in diameter (0.08 inches).
- Sand particles range from 0.05 to two millimetres (0.002 to 0.08 inches) in diameter.
- Silt particles range from 0.002 to 0.05 millimetres (0.00008 to 0.002 inches) in diameter.
- Clay particles are smaller than 0.002 millimetres in diameter (0.00008 inches).
These particles are made from various mineral types with different elemental composition, which affects the weathering processes and subsequent release of certain nutrients. Two soils with identical texture could be drastically different in fertility due to differences in mineral composition. For example, potassium content arises from mineral weathering in soil. Nitrogen, on the other hand, is not a constituent of any soil mineral.
The soil colloidal fraction refers to the microscopic particles of clay and organic matter. The surface of these particles is where most soil chemical reactions occur and it is very important in nutrient supply.
The proportion of sand, silt and clay determines the texture of a soil. Soil texture is grouped into five or more classes. Soil texture influences fertility by affecting moisture holding capacity, air exchange and cationA positively-charged ion (ion with a net positive charge). More exchange capacity (CEC). Since weathering is very slow in prairie soils, mineralogical composition is fairly uniform and, therefore texture can be a good indicator of CEC.
Adequate moisture is key to fertilizer response, and consequently also to achieving canola yield potential in western Canada. Research on the Prairies found that seed yield could vary from 1.8 to 3.3 kilograms per millimetre, which is equivalent to two to 3.7 bushels per inch of moisture.
Table 3. Seed yield (by location) per unit of water (at different precipitation probabilities) (kg mm-1)
One or more columns doesn't have a header. Please enter headers for all columns in order to proceed.
Table 3. Seed yield (by location) per unit of water (at different precipitation probabilities) (bu/inch)
One or more columns doesn't have a header. Please enter headers for all columns in order to proceed.
Source: Karamanos, R.E. and Henry, J.L. 2007. A Water Based System for Deriving Nitrogen Recommendations in the Canadian Prairies. Chapter 11, in. T. Bruuslema (ed.) Managing Crop Nitrogen for Weather. Proceedings of the Symposium “Integrating Water Variability into Nitrogen Recommendations” International Plant Nutrient Institute, Norcross, GA
The CEC is an important soil property that influences the storage of many plant nutrients in the soil. Most nutrients are present in the soil water as positively charged cations, and some as negatively charged anions. The CEC indicates a soil’s ability to hold or store cations. Prairie soil particles typically have a net negative charge. The process of electrical attraction that holds cations to negative surfaces of soil colloids is called adsorptionThe process of electrical attraction that holds cations to negative surfaces of soil colloids. More (not absorption). The cations are not permanently stuck to the colloidal surface and can be exchanged with other cations. A number of cations (such as potassium, magnesium, calcium, iron and aluminum) are part of the clay mineral structure and were determined by the soil forming from its parent material. Over time, certain cations may change into forms that are not easily removed from the exchange complexes. Adsorbed cations are not removed by water moving through the soil and can be accessed by plant roots. Cations with a higher positive charge (calcium, Ca+2, for example) are held more tightly than those with a lower charge (potassium, K+, for example).
All cations in soil are accompanied by a hydration cell (i.e., molecules of water), which affects the size and consequently the reactivity of cations. For example, potassium has a smaller hydration cell than calcium or sodium and reacts faster in soil.
Soil negative charges arise due to substitutions in the mineral crystals by elements with smaller positive charges, and due to reactions at the edges. The particle surface area proportion in a soil increases as the particle sizes get smaller. Therefore, a soil high in clay (which has a very small particle size) has a much greater surface area than a sandy soil (since sand has a large particle size).
The soil texture also impacts the charge of the soil. Clay minerals are microscopic layers of aluminum and silicon crystals formed by weathering of other minerals. Thus clays are called secondary minerals. The type of clay depends on the original minerals and the extent of weathering. Fairly young clays common in western Canada (such as montmorillonite) have a 2:1 arrangement of silica: alumina crystal sheets, while older clays have a 1:1 arrangement. Generally, 2:1 clays have 10 to 100 times more surface area, negative charges and, consequently, a higher CEC than 1:1 clays. Organic particles also contain a much higher number of negative charges than clay colloids. Therefore, soil organic matter levels greatly influence the CEC.
The CEC strongly influences soil fertility. A higher CEC means that more cations, including plant nutrients, can be loosely stored in a plant available form, giving the plant a greater pool of nutrients to draw from. There is a strong relationship between cations in the soil solution and those on the exchange phase of soil colloids: the more cations on the exchange phase, the more in the soil solution. That is why most of western Canadian soils that are rich in potassium, calcium and magnesium minerals have ample supply of available forms of these elements (nutrients). A high CEC also means that fewer cations will be lost through leaching below the root zone.
Since soils are predominantly negatively charged, anions [such as nitrate (NO3–) and sulphate (SO4-2)] are repelled by soil colloids and tend to stay in the soil water. They will flow with water and are more prone to leaching loss.
Soil organic matter plays an important role in soil fertility as a plant nutrient storehouse, especially since it is the only indigenous source of nitrogen in soils. Not only does organic matter adsorb many cations due to a high CEC, it also stores nutrients as part of its structure. As the organic matter is decomposed by soil microbes, nutrients are released from the organic structure into plant available forms by the process called mineralization. Mineralization from organic matter is the primary natural source of plant available nitrogen and sulphur in prairie soils. Mineralization also influences phosphorus availability with research showing that half of phosphorus availability comes from the mineralization of organic matter. Mineralization of individual nutrients will be described in later sections. Soil organic matter also improves physical properties such as water holding capacity, infiltration, aggregation (tilth) and buffering pH, which all impact soil fertility.